How digital signature approvals create a SOC/FDA/ISO compliant pull request review process
In this blog post, we’ll discuss how to use digital signature approvals to ensure your pull request review process meets all necessary compliance standards. Let’s get started!
Introduction to Digital Signature Approvals
Digital signature approvals have become an integral part of any modern enterprise workflow, especially those which involve compliance with industry standards such as Federal Drug Administration (FDA) 21 CFR Part 11 regulations, System and Organization Controls (SOC), and the International Standards Organization (ISO). Utilizing digital signature approvals during a pull request review process can help improve overall efficiency and ensure that all safety and quality requirements are met.
Digital signature approvals allow for consistent review of data across all stakeholders in a timely manner. Reviewers are able to digitally sign off on any changes without having to print out documents or files for comparison or signing. As long as there is an Internet connection, organizational users from any location can complete the workflow process quickly and easily. This helps to ensure that changes can be propagated quickly and properly throughout the system, reducing costly delays caused by document duplication or inefficiencies of manual paper processes.
Digital signature approvals also provide organizations with visibility into critical regulatory processes such as SOC compliance, 21 CFR Part 11 compliance, and ISO compliance so they can be sure they are meeting all relevant standards while providing quality service to their customers. Furthermore, it provides organizations with a clear audit trail so that they can trace requests throughout the entire process and identify areas where improvement may be needed.
By incorporating digital signature approvals into your organization’s workflow, you can reduce inefficiency due to manual document duplication and ensure high levels of regulatory compliance while improving customer service.
Implementing a Digital Signature Approval Process for Pull Requests
Digital signature approvals are quickly becoming reliable tools for organizations tasked with maintaining compliance standards in quality control and management of both internal and external personnel. Individuals that have direct contact with products meant to be released to markets under varying levels of oversight, such as those monitored by SOC, FDA, or ISO regulations, need to demonstrate a clear understanding of the processes outlined during the pull request review process.
Digital signature technologies can be used in combination with existing security measures, such as added layers of authentication, two-factor authorization, and encryption protocols to further simplify an organization’s ability to implement efficient and secure pull request review processes.
When integrating digital signature approvals into a pull request review process for compliance purposes, there are a few key considerations that should be taken into account:
- Authorization – Verify that authorizing personnel has the necessary credentials and access privileges required to approve requests on behalf of the organization.
- Security – Establish security controls that protect against unauthorized access while ensuring authenticated users abide by safety protocols set forth by relevant governing bodies.
- Auditing – Taking inventory of all assets associated with activities conducted during the pull request review period keeps organizations both informed and compliant when submitting documents.
- Workflows – Establishing effective workflows helps maintain accurate records while providing continuity in communication throughout all departments within an organization.
- Integration – Oversee integration between software systems, including third-party applications related to the pull request review process, for optimal visibility into all areas related to the subject matter.
- Best Practices – Adopting best practices such as periodic training outlines expected behavior from legitimate users attempting access without hindering IT efforts requiring additional repetitive scenarios from unrecognized users attempting repeat entry over time.
By following these considerations when implementing a digital signature approval process for requests related to SOC/FDA/ISO compliant structures, the organization can be sure their team is properly trained or scheduled for frequent retraining if needed. This ensures consistently secure practices associated with any elevated exchange data environments involving digital signatures today and in years moving forward.

The Role of Digital Signature Approvals in SOC Compliance
Digital signature approvals play an important role in helping organizations maintain compliance with the SOC framework. The SOC framework is designed to help organizations improve their data security and protect customer privacy. The digital signature approval process helps ensure that the right access controls and data protections are in place when processing pull requests.
For organizations to properly implement a compliant pull request review process, it is essential for them to have the proper access controls in place. Digital signatures can act as a form of secure access control by verifying a person’s identity and ensuring proper authorization before allowing them to process a pull request. This helps protect sensitive customer data from unauthorized changes or unauthorized users gaining access to sensitive information.
Digital signatures can also help automate SOC/FDA/ISO compliant processes, as they provide an efficient way of tracking and validating changes made within the organization’s system environment. In addition to enabling greater visibility on potential security-related issues that may arise before they become widespread problems.
Additionally, digital signature approvals help meet specific SOC requirements related to document authentication and integrity, which has become increasingly important with the growing number of data breaches occurring each year. Digital signatures provide an additional layer of security when handling sensitive documents, helping ensure that all changes are legitimate and can be tracked back to their original source or creator in case anything goes wrong.
Overall, digital signature approvals play an integral role in helping organizations adhere to all aspects of the SOC framework while improving visibility control across their system environment. By utilizing this powerful tool, companies can create greater trust between customers and the company, and reduce the risk of tampering with customer data or a corporate system breach resulting from hackers gaining access to unauthorized information.

The Role of Digital Signature Approvals in FDA Compliance
Digital signature approvals play a critical role in ensuring compliance with 21 CFR Part 11, which is a set of regulations issued by the US Food and Drug Administration (FDA) for electronic records and electronic signatures. These regulations apply to companies that are involved in the manufacture, distribution, or sale of drugs, medical devices, and other FDA-regulated products.
One of the key requirements of 21 CFR Part 11 is that electronic records and signatures must be as reliable and trustworthy as their paper counterparts. Digital signature approvals are an important way to meet this requirement, as they provide a secure and verifiable way to sign and approve electronic documents. When a document is digitally signed, a unique code is added to the document that serves as an electronic fingerprint of the signer. This code can be used to verify the identity of the signer and ensure that the document has not been tampered with.
Using digital signature approvals in the pull request review process can help to ensure compliance with 21 CFR Part 11 by providing a secure and verifiable way to sign and approve electronic documents related to product development, testing, and production. By implementing a system for digital signature approvals, companies can demonstrate to the FDA that they are taking appropriate measures to ensure the integrity and reliability of electronic records and signatures. This can help to reduce the risk of non-compliance and protect against the potential penalties and fines associated with 21 CFR Part 11 violations.
The Role of Digital Signature Approvals in ISO Compliance
Digital signature approvals can be an essential part of ensuring that a business is compliant with requirements dictated by agencies such as the FDA, ISO, and SOC. These regulations can often have specific processes that must be followed to ensure consistent handling. In these cases, digital signature approvals are needed to ensure that all documents are properly documented and signed, which can help with compliance.
A digital signature is the electronic equivalent of a physical signature. Digital signatures use computer cryptography to verify the validity of users’ claims about who they are and what documents they have signed or authorized. To be considered digitally valid for most legal purposes, it must include two components – proof of identity (the individual’s identification details) and proof of intent (their commitment to the process).
In order for a pull request review process to be compliant with ISO standards and regulations, all associated documents must be properly approved via digital signatures. This helps provide proof that any changes made follow the acceptance criteria outlined in the requirements document related to the pull request process. Additionally, digital signature approvals help preserve transparency by providing an easy-to-follow audit trail containing user information at each step in the process.
Digital signature approvals also improve reviews by adding extra layers of verification – for example, two different individuals may need to approve a policy or change before it is implemented; this ensures that there is a double assurance from different sources on any applicable changes made. In essence, digital signatures help formalize reviews and provide higher levels of accuracy and consistency when measuring compliance rates against agency expectations such as ISO regulations or FDA quality standards.Create Digital Signature Approval Token in Workzone for Bitbucket Cloud
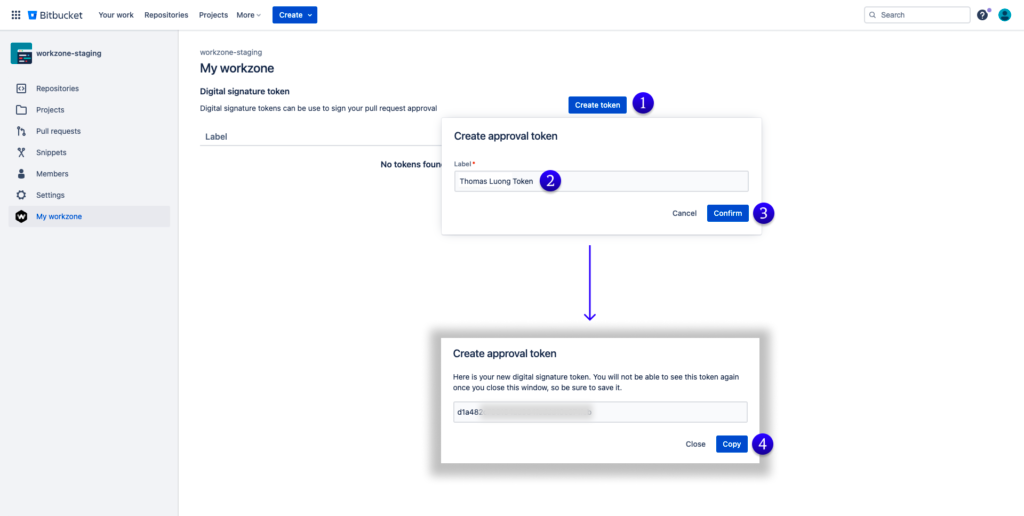
Common Challenges and Solutions in Implementing Digital Signature Approvals
In today’s ever-evolving regulatory landscape, it is essential for organizations to have an efficient and effective approach to software development and change management processes, including pull request review. The use of digital signatures provides a way for organizations to operationalize their process and more easily conform to applicable regulations. However, digital signature approvals can sometimes come with their own set of challenges that must be addressed in order to ensure a smooth transition into a compliant pull request review process.
One of the main challenges associated with transitioning towards digital signature approvals is the lack of user familiarity with the technology. Organizations must address this challenge by providing clear, concise training materials that explain what a digital signature is and how it works. In addition, users should be given adequate practice time in order to become comfortable navigating the system and ensure that they fully understand how their duties are related to pull request reviews.
Another challenge organizations may experience during a transition toward digital signature approvals lies in getting all stakeholders on board with the new process. It’s important for those leading the initiative to make sure everyone involved understands why implementing this new process is beneficial for both organizational efficiency and compliance purposes. In addition, these leaders should strive for transparency throughout the entire roll-out by providing frequent updates on progress and communicating any difficulties encountered along the way so that all users feel informed about every step taken throughout this process.
When used properly, digital signatures offer efficient, secure and scalable ways for organizations to implement compliant pull request reviews. By addressing common challenges such as user familiarity, organization-wide awareness, data accuracy and security protocols upfront, companies will be able to successfully transition into an effective SOC/FDA/ISO compliant process governed by digital signature approvals.Digitally Sign Pull Requests with Workzone for Bitbucket Data Center/Server
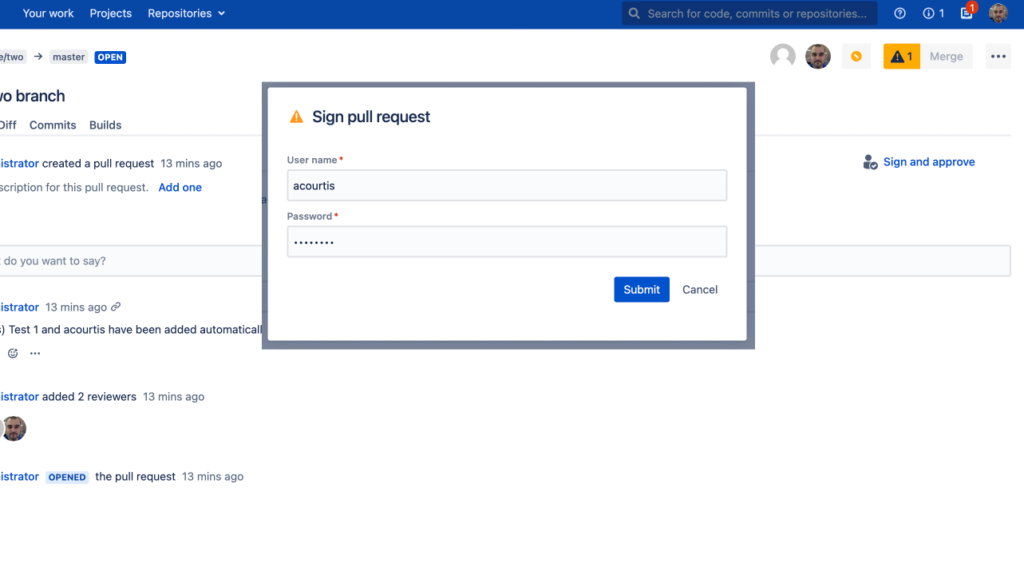
Real-World Examples of Digital Signature Approvals in Pull Request Review Processes
Digital signature approvals speed up the pull request review process, making it easier and more streamlined to implement a secure and compliant review process. Digital signatures also help organizations comply with regulatory requirements such as SOC, FDA or ISO.
This article takes a look at real-world examples of digital signature approvals being implemented in pull request review processes. We will examine case studies and best practices for implementing digital signatures in pull request review processes.
The first example is from a medical device company that was looking to implement an efficient and secure process for reviewing pull requests from its software engineering team. The company uses the Workzone for Bitbucket tool in its merge control process, to utilize the digital signature functionality to track when changes have been approved, ensuring compliance with regulatory requirements while speeding up the time it took for reviews. The company also utilized the workflow automation elements of Workzone, which integrate with their source code repository so they can route Pull Requests through the right channels and immediately know when they have been approved or rejected. Workzone also automatically adds the relevant reviewers that need to sign the pull request, and thus enforcing consistent compliance requirements.
Another example comes from a financial services company that needed an audit trail of all the changes made in their source code during a period of time. Using digital signatures during their pull request review process allowed the company to create an automated audit trail of each modification. This was to ensure compliance with ISO regulations that require developers’ changes to be reviewed by multiple individuals throughout different stages of the development cycle.
These are just some examples where digital signature approvals are helping organizations improve their pull request review processes while maintaining compliant security protocols across different industry standards, including SOC, FDA, ISO etc. Organizations planning on implementing digital signature approvals into their own processes can take cues from these case studies while considering which workflow automation platforms best fit their use cases, as well as how many stages must be included in each pull request workflow depending on existing industry regulations which must be adhered too at all times.
Conclusion
In this article, we discussed how digital signature approvals can help to implement a SOC/FDA/ISO compliant pull request review process. We explored the pros and cons of using digital signatures and key principles to consider while developing SOC/FDA/ISO compliant pull requests. We provided recommendations on how to ensure these documents are compliant with the standard by using an automated system of digital signatures.
Finally, we discussed trends for implementing digital signature requirements in regulated industries, such as Medical Device Manufacturing and financial services, and how these companies utilize tools such as Workzone for Bitbucket to fulfil their requirements. These regulations are constantly evolving, and companies need to be swift in their implementation towards achieving compliance with SOC, FDA 21 CFR Part 11 and ISO standards.
Digital signature admittance is here to stay and provides many opportunities for organizations worldwide to make timely decisions through obtaining secure evidence regarding decision-making processes across government bodies, businesses, research institutions and beyond. The immediate goal is that, by following the best practices outlined above, combined with new cutting-edge technology, we are closer to achieving successful compliance goals at every level within any organization.
Izymes provides the tools to make your software development and change management process compliant, more effective and easy to implement. Visit our apps on the Atlassian Marketplace
Want to see Workzone Digital Signatures in action? Book a demo here